|
|
10 modules to calculate YOUR problem with a step-by-step solution
|
a. Interconversion
This module calculates any of three values: pH, pOH, [H+] and [OH-], if any one of them is known.
b. Weak Acid/Base Dissociation
This module can perform the following calculations:
- Known initial concentration of a weak acid and Ka, calculate percent dissociation and pH.
- Known initial concentration of a weak acid and percent dissociation, calculate Ka and pH.
- Known initial concentration of a weak base and Kb, calculate pH.
c. Weak Acids Mixture
This module calculates the pH of a solution containing two weak acids of very different strengths.
d. Salt Producing Basic Solutions
This module calculates the pH of the salts that produce basic solutions.
e. Salt Producing Basic Solutions
This module calculates the pH of the salts that produce acidic solutions.
f. Buffered Solutions (Acid/Salt) and pH Changes
This module calculates the pH of buffered solutions and the pH changes in the solution when a strong base is added.
g. Buffered Solutions (Base/Salt) and pH Changes
This module calculates the pH of buffered solutions and the pH changes in the solution when a strong acid is added.
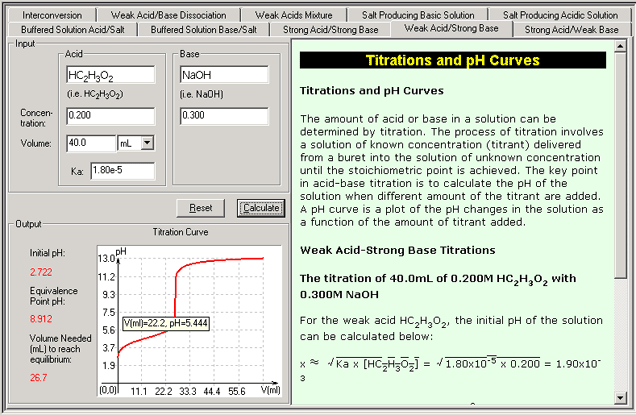
Titration and pH Curvers
h. Strong Acid-Strong Base
i. Weak Acid-Strong Base
j. Strong Acid-Weak Base
These three modules are for three types of titrations. By choosing a titrant of known concentration and an unknown solution, the key numbers (initial pH, pH at the equilibrium point and the volume needed to get the equilibrium) and pH curves can be calculated.