Basic Concept
Standard Enthalpy of Formation (ΔHfº) of a substance is defined as the change in enthalpy that accompanies for formation of one mole of a substance from its elements with all substances in their standard states. The process has been carried out under standard conditions (normally 25°C, 1 atm).
The enthalpy change of chemical reaction can be calculated using the ΔHfº of the substances involved in the reactionas shown in the following formula:
ΔHºreaction = ΣnpΔHfº(products) - ΣnrΔHfº(reactants)
where the first Σ to take sum of all the products in this reaction, and the second sum all the reactants. The np and nr represent the moles of each product or reactant, respectively.
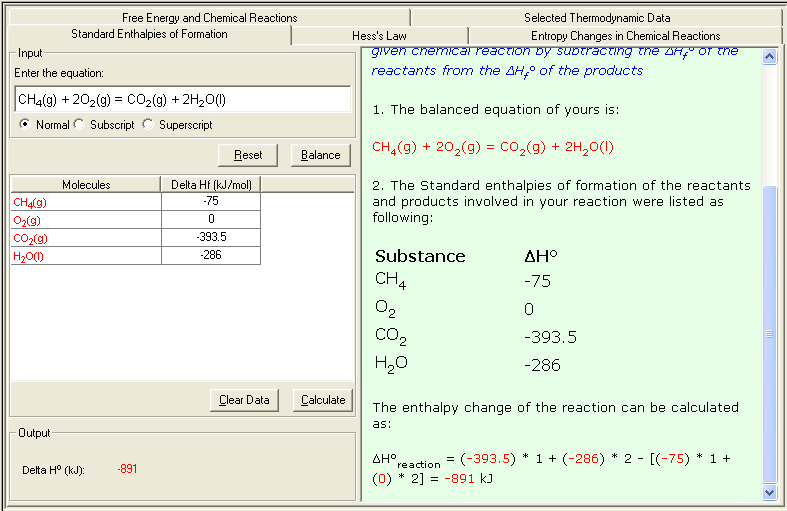
Example: calculate the enthalpy change of the following reaction:
CH4(g) + 2O2(g) = CO2(g) + 2H2O(l)
From the Table of Thermodynamics Data, you can obtain the Standard Enthalpies of Formation of the substances appeared in above reaction:
| Substances |
ΔHfº (kJ/mol) |
| CH4(g) |
-75 |
| O2(g) |
0 |
| CO2(g) |
-393.5 |
| H2O(l) |
-286 |
The enthalpy change of the reaction can be calculated as:
ΔHº = [(-393.5) + (-286) * 2] - [(-75) - (0) * 2] = -891 kJ
User Instructions
For the above example,
1. Select Standard of Enthalpy of Formation link either from the front page (Table of Content) or from the Thermochemistry module. The Input and Output screen appears.
2. In the Input area, enter the problem equation and click Balance button to balance the equation; then enter the Delta H value (ΔHfº) for each molecule. You may consult the Selected Thermodynamic Data sub-module for reference data.
3. Click Calculate to output the answer with the detailed step-by-step show work on the side as shown below: