Stoichiometric factors relate the molar amounts of any 2 substances in a chemical reaction.
When there is too much of one reactant to react completely with a given amount of a second reactant, the first reactant is said to be "in an excess".
When there is too little of a reactant to react completely with a given amount of a second reactant, the first reactant is said to be a "limiting reagent".
When both reactants are completely consumed the reactants are said to be in "stoichiometric proportions"
This module provides the stoichiometric calculations on any given reaction.
In addition to the problem solving module, the "Show Work" is also displayed along with the solution to illustrate the step-by-step guide in how your problem has been solved.
This is one step process, enter the known data and press Calculate to output the unknowns.
1. Select it from the front page. The Input and Output screen appears.
2. Enter the balanced or unbalanced equation.
Here is the typing instruction:
To enter normal text, just type in as is, be sure to use both cases for element symbols, e.g. Fe (Not FE). Be sure the Input Option - Normal is selected (default).
To enter the equation sign, you can use either "=" or "--->"or "<--->" symbols.
To enter superscripts for the charge species (Ca2+), there are two ways to input them:
Option 1: Select Superscript in the Input Option and type in ONE character, the option selection will return back to Normal. Click Superscript again, and enter the second superscript character. For example, the keystroke sequence to enter Ca2+ is C-a-"Superscript"-2-"Superscript"-"+".
Option 2: Press UpArrow key, the enter a superscript character, if needed, press UpArrow key again and then type in the second superscript character. For example, the keystroke sequence to enter Ca2+ is C-a-UpArrow-2-UpArrow-"+".
To enter subscripts for atom counters (H2), follow the similar instructions above, but use Subscript option box or DownArrow.
To enter a hydrated compound (CuSO4•5H2O), type in "." (period). The period (.) will be converted to the dot symbol(•) automatically upon typing in.
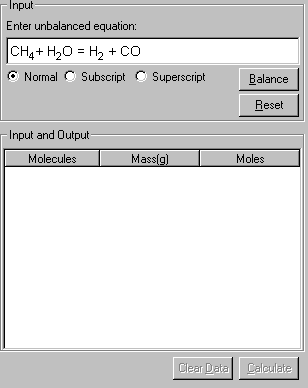
3. Upon entering the equation, click Balance. The equation is balanced, and the balanced equation is placed in the Input field. The individual species in the equation is listed in the Input and Output table below.
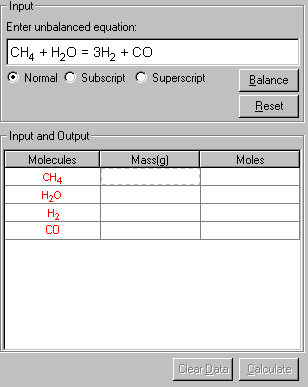
4. Enter all the known conditions given from the problem in the Mass and Moles columns. Click the cell and type in the data. In this case, only the mass of CH4 is given.
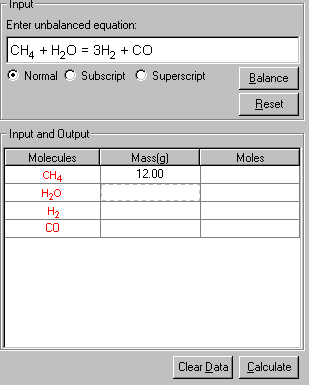
5. Finally, click Calculate to output the results in red.
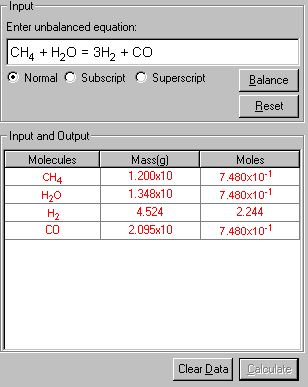
Both the stoichiometric mass and moles of each species are shown in the table.
The "Show Work" page of this problem is now displayed side-by-side with the solution in explaining how your problem has been solved in the step-by-step fashion.
If the mass or moles of more than one species are entered, the program will output the results based on the limiting reagent.
5. To restart, click Clear Data.