The empirical formula for a compound is the simplest formula that shows the ratio of the numbers of atoms of each kind in the compound, e.g. for hydrogen peroxide H2O2, its empirical (simplest) formula is HO.
A molecular formula shows the number of atoms of each kind in a molecule. Therefore, the molecular formula of a compound is the multiples of empirical formula, e.g. hydrogen peroxide's molecular formula is H2O2.
By entering the percentages or the mass of elements in a compound and the weight of it, optionally, the module calculates the empirical formula/weight and molecular formula/weight if the molecular weight is given.
In addition to the problem solving module, the "Show Work" is also displayed along with the solution to illustrate the step-by-step guide in how your problem has been solved.
This is one step process, enter the known data and press Calculate to output the unknowns.
1. Click the Molecular Formula tab or select Empirical and Molecular Formula from the front page. The Input and Output screen appears.
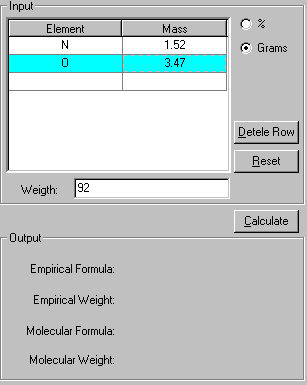
2. If the percentages of the elements are given, click the % check box. If the grams of each element is given, click the Grams check box. Enter the data for the element. In this case, enter the mass of N and O atoms in the compound and its molecular weight.
3. Click Calculate to output the results in red.
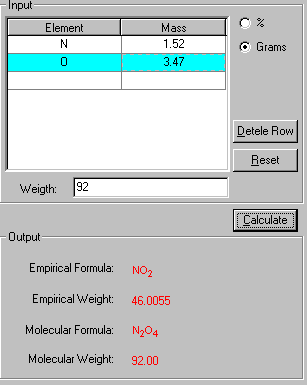
The "Show Work" page of this problem is now displayed side-by-side with the solution in explaining how your problem has been solved in the step-by-step fashion.
4. Click Reset to do another problem.