Titrations
The amount of acid or base in a solution can be determined by titration.The process of titration involves a solution of known concentration (titrant) delivered from a buret into the solution of unknown concentration until the stoichiometric point is achieved. The key point in acid-base titration is to calculate the pH of the solution when different amount of the titrant are added. A pH curve is a plot of the pH changes in the solution as a function of the amount of titrant added.
There are three types of Acid-Base titration:
The millimole (abbreviated mmol) is very common unit used in titrations:
mmol = Molarity x mL solution = 10-3 mol
This module is for one of the three possible cases of titration. By choosing a titrant of known concentration and an unknown solution, the key numbers (initial pH, pH at the equilibrium point and the volume needed to get the equilibrium) and pH curves can be calculated.
Titrations of Weak Bases with Strong Acids
In the titration of 100.0mL of 0.050M NH3 with 0.100M HCl (Kb = 1.8 x 10-5),
Initial moles of OH- = 0.050M x 100.0mL = 5.0mmol
x2 (Kb x 0.050) = 9.0 x 10-7 and x 9.49 x 10-4
pH = 14.0 + log(x) = 10.98
Initial moles of NH3 = 5.0mmol
Added H+ moles = 50.0mL x 0.100 M = 5.0mmol
At this point, all the original NH3 has been converted to NH4+.
[NH4+]0 = 5.0mmol / (100.0mL + 50.0mL) = 0.033M
[H+]0 0
[NH3]0 0
Equilibrium
[NH4+] = 0.033M - x
[H+] = x
[NH3] = x
Ka = Kw /Kb = [NH3] [H+] / [NH4+] = x2 / (0.033 - x) x2 / 0.033 = 5.6 x 10-10
x = 4.32 x 10-6
pH = - log([H+]) = 5.37
This is one step process, enter the known data and press Calculate to output the unknowns.
1. Select Strong Acid/Weak Base link from the front page or Strong Acid/Weak Base tab from the Acid, Baseand Salt module. The Input and Output screen appears.
2. In the Input area, enter the known quantities with a proper significant figure with the above example.
3. Click Calculate to output the answer.
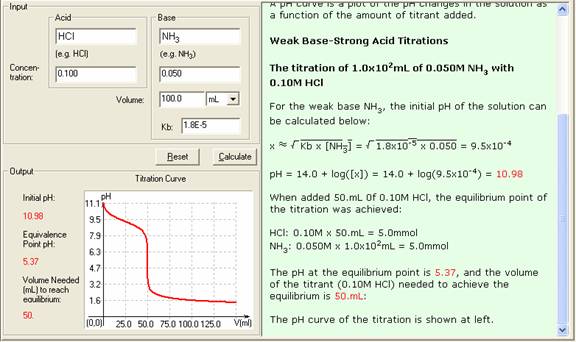
4. The Show Work area on the right shows you step-by-step how your problem has been solved.
To start a new problem, click Reset. All Input fields will be cleared. Follow Step 1-3 again.