When a salt dissolves in water, it breaks up into its ions. Under certain conditions, these ions can behave as acids.
This module calculates the pH of the salts that produce acidic solutions.
For example, in an aqueous solution of NH4Cl, the major species are
NH4+, Cl- and H2O
The NH4+behaves as a weak acid:
NH4+(aq) = NH3 (aq) + H+ (aq)
The Cl- ion does not affect the pH of the solution.
Ka = [NH3][H+] / [NH4+] (1)
The Kb of NH3 is 1.8 x 10-5,
Ka x Kb = Kw = 1.0 x 10-14 (2)
Thus
Ka = Kw / Ka = 1.0 x 10-14 / 1.8 x 10-5 = 5.6 x 10-10 (3)
Using Ka or its formulae (1) the pH scale of the aqueous solution of NH4+can be calculated.
In the 0.10 M NH4+ solution,
Ka = 5.6 x 10-10 = [NH3][H+] / [NH4+] = (x)(x) / (0.10 - x) x2 / 0.10
x 7.5 x 10-6
pH = 5.13
For the above example, in the 0.10 M NH4Cl solution(Kb = 1.8 x 10-5 ).
This is one step process, enter the known data and press Calculate to output the unknowns.
1. Select Salt Producing Acidic Solution link from the front page or Salt Producing Acidic Solution tab from the Acid, Baseand Salt module. The Input and Output screen appears.
2. In the Input area, enter the known quantities with a proper significant figure.
3. Click Calculate to output the answer.
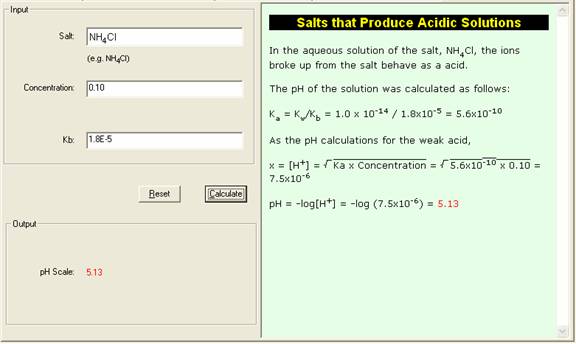
4. The Show Work area on the right shows you step-by-step how your problem has been solved.
To start a new problem, click Reset. All Input fields will be cleared. Follow Step 1-3 again.