A buffered solution is one that resists change in pH when either hydroxide ions or protons are added. A buffered solution may contain a weak acid and its salt or a weak base and its salt.
This module calculates the pH of buffered solutions and the pH changes in the solution when a strong acid is added.
1. pH of a buffered solution
In a buffered solution example that contains 0.25M NH3 (Kb = 1.8 x 10-5) and 0.40M NH4Cl, the equilibrium is:
NH3(aq) + H2O = NH4+ (aq) + OH- (aq)
Kb = [NH4+][OH-] / [NH3] = 1.8 x 10-5
The concentrations are as follows:
NH3(aq) = N H4+ (aq) + OH- (aq)
Initial: 0.25 0.40 0
Change: -x x x
Equilibrium: 0.50 - x 0.40 + x x
Kb = 1.8 x 10-5 = [N H4+][OH-] / [NH3] = (0.40 + x) (x) / (0.25 - x) (0.40) (x) / 0.25
x 1.1 x 10-5
pOH = 4.95
pH = 14.00 - 4.95 = 9.05
2. pH changes in buffered solutions
When 0.10 mol gaseous HCl is added to 1.0L the buffered solution, the stoichiometry is shown as follows:
NH3 + H+ == NH4+
Before reaction: 0.25 mol 0.10 mol 0.40 mol
After reaction: 0.15 mol 0 0.50 mol
The equilibrium of the solution can be shown in a shorthand notation:
NH3 + H+ == NH4+
Initial: 0.15 0 0.50
Change: -x x x
Equilibrium: 0.15 - x x 0.50 + x
Ka = Kw / Kb = 5.6 x 10-10 = [H+][NH3] / [NH4+] = (x) (0.15 - x) / (0.50 + x) (x)(0.15) / 0.50
Thus
x 1.9 x 10-9
[H+] = x = 1.9 x 10-9 M
pH = 8.73
The change in pH by adding 0.10 mol H+ to this buffered solution is only
8.73 - 9.05 = -0.32
If 0.10 mol HCl is added to 1.0L water, the pH will from 7.00 to 1.00 (-6.00).
This is two step processes, enter the known data and press Calculate to output the unknowns.
1. Select Buffered Solution Base/Salt link from the front page or Buffered Solution Base/Salt tab from the Acid, Baseand Salt module. The Input and Output screen appears.
2. In the Input area, enter the known quantities with a proper significant figure with the above example.
3. Click Calculate to output the answer.
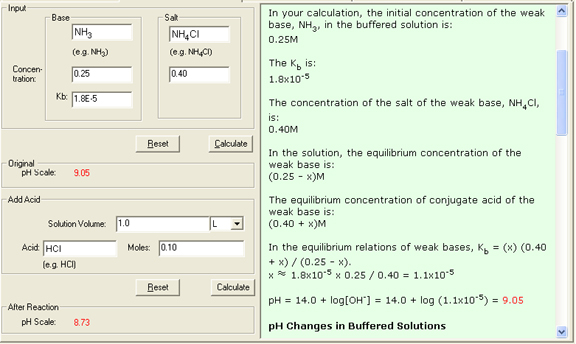
4. The Show Work area on the right shows you step-by-step how your problem has been solved.
To start a new problem, click Reset. All Input fields will be cleared. Follow Step 1-3 again.