Solution Stoichiometry - The study of the quantitative relationships in a reaction among reactants and products.
This module provides a step-by-step solution for stoichiometric problems.
1. Select Stoichiometry link from the front page or Stoichiometry tab from the Solution module. The Input and Output screen appears.
2. In the Input area, enter the known quantities with a proper significant figure. Select the units associated with the input.
This is a two step process,
first enter and Balance the equation for the reaction. For example:
BaCl2(aq) + K2CrO4(aq) = BaCrO4(s) + 2KCl(aq)
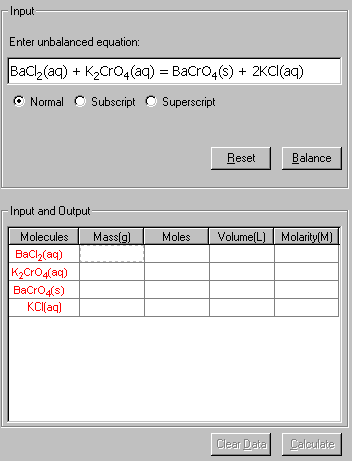
then, input the known quantities: volume (1.050 L) and Molarity (0.470) of BaCl2, and the 300.0 g of K2CrO4.
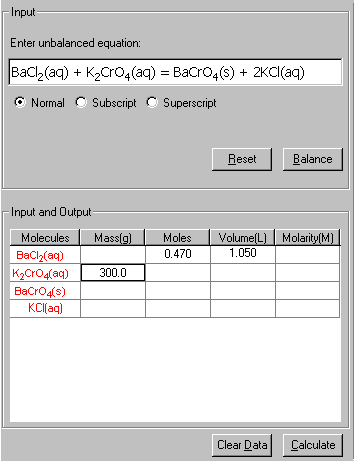
3. Click Calculate to output the answer.
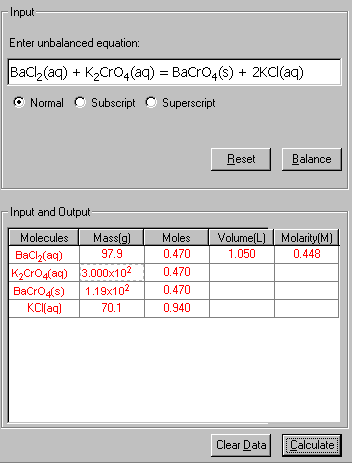
4. The Show Work area on the right shows you step-by-step how your problem has been solved.
To start a new problem, click Reset. All Input fields will be cleared. Follow Step 1-3 again.