Boiling-Point (Tb) - The temperature at which the vapor pressure of a liquid equals atmospheric pressure.
Freezing-Point (Tf) - The temperature at which the rate of liquid converting to solid equals the rate of solid converting to liquid.
Boiling-Point Elevation (ΔTb) - The increase in the boiling point for a solution than the pure solvent.
Freezing-Point Depression (ΔTf) - The decrease in the freezing point for a solution than the pure solvent.
For dilute solutions, the increase in boiling point, and the decrease in the freezing point depend on the molality of the solute in the solution:
ΔTb = Kb x msolute
ΔTf = Kf x msolute
Where Kb and Kf are the boiling-point elevation constant and the freezing-point depression constant, respectively.
This module calculates Boiling-Point Elevation or Freezing-Point Depression of a solution by knowing its molality, or calculate other related quantities.
Example Question: A solution was prepared by dissolving 27.00 g solute to 225.0g water. The forming solution had a boiling point of 100.34 °C, calculate the molecular weight of the solute molecule.
1. Select Boiling Point Elevation and Freezing Point Depression link from the front page or the tab from the Solution module. The Input and Output screen appears.
2. In the Input area, enter the known quantities with a proper significant figure. Select the units associated with the input.
3. Click Calculate to output the answer.
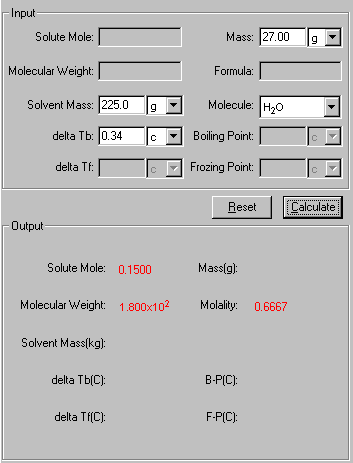
4. The Show Work area on the right shows you step-by-step how your problem has been solved.
To start a new problem, click Reset. All Input fields will be cleared. Follow Step 1-3 again.