The formula weight of a compound is the sum of all the atomic weights of the elements present in the formula of the compound. Some text also refers it to formula mass.
Atomic weight is the average atomic mass of each element (in amu - atomic mass unit). Strictly speaking, one should use the term atomic mass, but the term atomic weight is now usually being used instead.
e.g. The formula weight of H2O = 2x Atomic Weight of H (2x1.008) + Atomic Weight of O(15.999) = 18.015
Formula weight of molecular compound is often called molecular weight. In chemistry, mass and weight are commonly used interchangeably.
Mass is the amount of a substance in grams, also called weight.
Mole is a quantity of a substance equal in weight to its formula weight, in moles (mol).
therefore, number of moles = Mass / MW
Avogadro's number is defined as the number of units in a mole, which has been found experimentally to be 6.022 x 1023molecules/mol. A package of 6.022x1023 particles is one mole.
The number of molecules = Moles x 6.022 x 1023
With input of a molecular formula and mass, moles, or number of molecules of the compound, this module outputs the molecular weight, moles, the number of molecules, mass percentages of each atom.
In addition to the problem solving module, the "Show Work" is also displayed along with the solution to illustrate the step-by-step guide in how your problem has been solved.
This is one step process, enter the known data and press Calculate to output the unknowns.
1. Select The Mole and Molecular Weight from the front page. The Input and Output screen appears.
2. Under Input Molecular Formula, enter the formula of the compound. Optionally, you can also enter the mass or moles or number of molecules of this compound.
Here is the typing instruction:
To enter normal text, just type in as is, be sure to use both cases for element symbols, e.g. Fe (Not FE). Be sure the Input Option - Normal is selected (default).
To enter subscripts for atom counters (H2), follow the similar instructions above, but use Subscript option box or DownArrow.
To enter a hydrated compound (CuSO4•5H2O), type in "." (period). The period (.) will be converted to the dot symbol(•) automatically upon typing in.
3. Click Calculate to output the results in red.
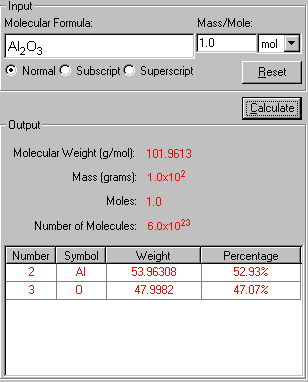
The "Show Work" page of this problem is now displayed side-by-side with the solution in explaining how your problem has been solved in the step-by-step fashion.
4. To restart, click Reset.