The Van der Waals equation is an equation similar to the Real Gas Law, but includes two constants, a and b, to account for deviations from ideal behavior.
The van der Waals equation is:
[P + (n2a/V2)](V - nb) = nRT
Where:
P - pressure,
V - volume,
n - number of moles,
T - temperature,
R - ideal gas constant. If the units of P, V, n and T are atm, L, mol and K, respectively, the value of R is 0.0821
a and b - constants, which are chosen to fit experiment as closely as possible to individual gas molecule.
When the volume, temperature and the number of moles of the gas molecule are known, the pressure can be calculated:
P = [nRT/(V - nb)] - n2a/V2
To calculate Volume:
To calculate the volume of a real gas, V in term n2a/V2 can be approximated as: nR/TP
as ideal gas. Then, V can be calculated as:
V = nR3T3/(PR2T2+aP2) + nb
The van der Waals constants a and b of molecular N2 is 1.390000 and 0.039100, respectively.
To calculate Pressure:
According to the van der Waals equation,
P = nRT/(V - nb) - n2a/V2
Example:
P = 1.500 x 0.0821 x 3.000x102 / (2.000 - 1.500 x 0.039100) - (1.500)2 x 1.390000 /(2.000)2
= 1.825x10 atm
The force exerted per unit area of surface, typical pressure units are ATM, mmHg and kPa.
The measurement of space taken by a substance, it is length cubed, typical units are L, mL and m3.
A measure of the average kinetic energy of the particles in a sample of matter, expressed in terms of units or degrees designated on a standard scale. Typical units are K, F and C.
The mass of the object divided by its volume. Typical units are g/mL and kg/m3.
The formula weight of a compound is the sum of all the atomic weights of the elements present in the formula of the compound. Some text also refers it to formula mass. Typical unit is g/mol.
Mass is the amount of a substance in grams, also called weight.
The constant that appears in the ideal gas equation (PV=nRT). It is usually expressed as 0.08206 L x atm/K x mol or 8.314 J/K x mol.
The number of moles of solute dissolved in one kilogram of solvent.
The number of moles of solute in one liter of solution.
This module is to compute pressure or volume of a gas according to the van der Waals equation.
In addition to the problem solving module, the "Show Work" is also displayed along with the solution to illustrate the step-by-step guide in how your problem has been solved.
This is an one-step process, enter the known data and press Calculate to output the unknowns for each process
1. Select Real Gases - Van der Waals Equation link from the front page or Real Gas tab from the Gases module.
2. First enter three of the four quantities Temperature, Pressure, Moles and Volume with proper significant figures and units.
3. Select the gas molecule from the Gas drop-down list.
4. Click Calculate. The answer is displayed in the Output field.
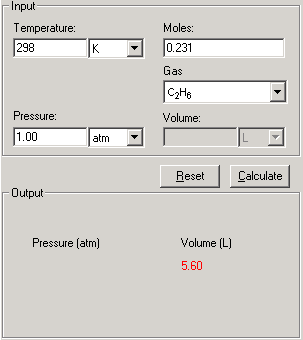
5. The Show Work area on the right shows you step-by-step how your problem has been solved.
To start a new problem, click Reset. All Input fields will be cleared. Follow Step 1-4 again.