Electronegativities, Bond Polarities and Dipole Moments -The electrons on the most chemical bonds are unequally shared. The reason of the inequality is that the atoms forming bond have different ability to attract electrons. Electronegativity is a measure of the relative attraction that an atom has for the shared electrons in a bond. Bond Polarity is a measure of inequality in the sharing of bonding electrons. The Bond Polarity is a vector, pointing from the atom with less electronegativity to the atom with larger one. The separation of positive and negative charges causes an electric dipole moment. If the vector sum of Bond Polarities of a molecule is not zero, the molecule is said having Dipole Moment.
This module is to predict whether a given molecule has a non-zero dipole moment and the directional bond polarities of all bonds to a central atom based on the electronegativities of the associated atoms.
This is an one-step solution. The 3D structure of a molecule and the information about the dipole moment (Yes/No) are displayed. The electronegativity of each atom in a molecule is shown and from this data, the direction (in arrow) of the polarities of each bond is displayed.
1. Click Dipole tab to open Dipole/Bond Polarity module.
On the left pane, it is the Compute module and one the right it is the Guide page. The top pane is the navigational toolbar.
2. Enter the formula of a molecule from Input field - Enter a Molecular Formula, in this case, NO3-.
Note: To enter subscript and superscript, use the Subscript and Superscript options below the input box. Alternatively, you can use <Up Arrow> to enter superscript and <Down Arrow> to enter subscript. The keyboard sequence to enter NO3- is N-O-<Down Arrow>-3-<Up Arrow>-"-".
3. Click Calculate to proceed. If you need to restart, click Reset.
The Dipole screen appears.
It displays the 3D structure of the input molecule. The electronegativity of each atom is listed. From the difference of the bonded pair's electronegativities, the bond polarity is determined, the polar arrow is pointed to the atom with higher electronegativity value. The dipole moment information is shown (whether the molecule has a dipole).
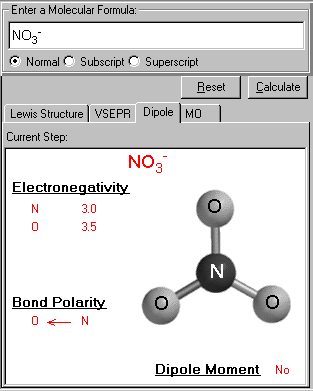
Bond Polarity is shown here, the arrow pointing to the negative charge center (or larger electronegativity) with the tail of the arrow indicating the positive center of the charge (or lower electronegativity).
In this case of NO3-, due to the three equivalent resonance structures (the same N-O bond) and trigonal planar geometry, all bond polarities are cancelled out. The net dipole moment is zero.