Binary Compounds - They are the compounds formed from the metal cation and the nonmetallic anion, such as sodium chloride - NaCl. Most ionic compounds are binary compounds, or compounds formed from just two elements. The naming conversion is such that the anion is named by taking the first part of the element name (chlorine) and adding -ide.
The Guidelines of forming binary ionic compounds:
Each compound must be electrically neutral - that is the sum of charges on the cation and the anion in each formula unit must add up to zero. Therefore, the subscript of the cation is numerically equal to the charge on the anion, vice versa.
This module is to display the formula and the name of any binary compound by selecting the cation and anion of the compound.
This is an one-step solution. Both the formula and name of the formed compound are shown.
1. Click Binary tab to open Binary Compound module.
2. Select both Cation and Anion from their drop-down lists in the Input field.
3. Click Calculate to proceed. If you need to restart, click Reset.
The formula and name of this binary compound are shown.
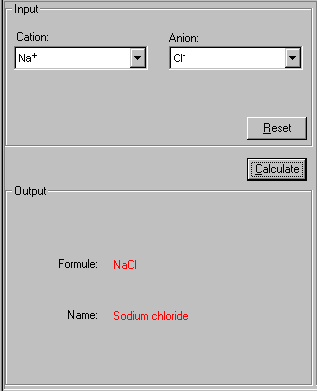
In this case, the cation Na+ and anion Cl- make a binary compound called NaCl with the name sodium chloride.